This soft, electronic ‘nerve cooler’ could be a new way to relieve pain. The device uses evaporative cooling to block pain signals, experimented successfully on rats.
After injury due to an accident or medical procedure, various forms of pain relief may be required. These can include analgesic medications or local injections to dull the pain receptors, but may also be as simple as applying something cold to the location causing the pain, such as ice packs for sore or bruised joints or muscles.
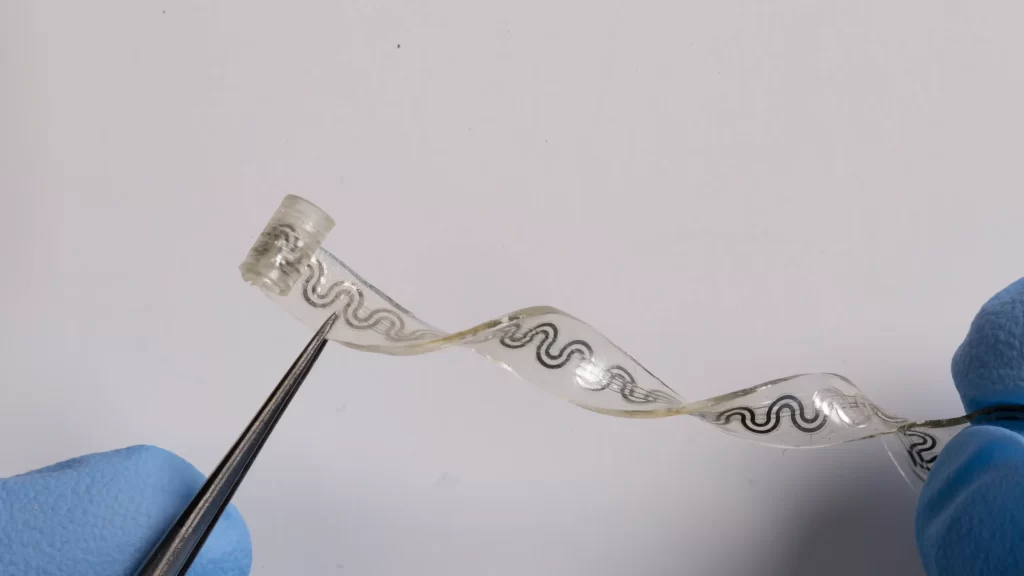
Reeder et al. developed a soft, miniaturized, implantable cooler to temporarily block nerve conduction using a liquid-to-gas phase transition as the cooling mechanism (see the Perspective by Jiang and Hong). They borrowed the design of electrical nerve cuff and substituted electrical wires with a microfluidic channel carrying a microliter volume of bioinert coolant. A thermal thin-film sensor integrated within the cuff enabled monitoring of the temperature in real time, thus enabling closed-loop control. —MSL

A flexible electronic implant could one day make pain management a lot more chill.
Created from materials that dissolve in the body, the device encircles nerves with an evaporative cooler. Implanted in rats, the cooler blocked pain signals from zipping up to the brain, bioengineer John Rogers and colleagues report in the July 1 Science.
Though far from ready for human use, a future version could potentially let “patients dial up or down the pain relief they need at any given moment,” says Rogers, of Northwestern University in Evanston, Ill.
Implantable devices capable of targeted and reversible blocking of peripheral nerve activity may provide alternatives to opioids for treating pain. Local cooling represents an attractive means for on-demand elimination of pain signals, but traditional technologies are limited by rigid, bulky form factors; imprecise cooling; and requirements for extraction surgeries. Here, we introduce soft, bioresorbable, microfluidic devices that enable delivery of focused, minimally invasive cooling power at arbitrary depths in living tissues with real-time temperature feedback control.
Construction with water-soluble, biocompatible materials leads to dissolution and bioresorption as a mechanism to eliminate unnecessary device load and risk to the patient without additional surgeries. Multiweek in vivo trials demonstrate the ability to rapidly and precisely cool peripheral nerves to provide local, on-demand analgesia in rat models for neuropathic pain.







